moles of an element in a compound calculatortoxic chemicals in the environment ppt
The measure used to compare quantities of atoms is the mole. WebThe molar mass of KClO3 is 122.548 g/mol. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. How does significant figures work, like how do I know to round to 2 or 3? "I've been stuck on a chemistry problem for hours, this article was easy to follow along with and really cleared, "I think is one of the best helpers of all, thank you so much. This gives a molar mass of 126.737 Analytical cookies are used to understand how visitors interact with the website. Find: Empirical formula \(= \ce{Fe}_?\ce{O}_?\), \[69.94 \: \text{g} \: \ce{Fe} \nonumber \], \[30.06 \: \text{g} \: \ce{O} \nonumber \], \[69.94 \: \text{g} \: \ce{Fe} \times \dfrac{1 \: \text{mol} \: \ce{Fe}}{55.85 \: \text{g} \: \ce{Fe}} = 1.252 \: \text{mol} \: \ce{Fe} \nonumber \], \[30.06 \: \text{g} \: \ce{O} \times \dfrac{1 \: \text{mol} \: \ce{O}}{16.00 \: \text{g} \: \ce{O}} = 1.879 \: \text{mol} \: \ce{O} \nonumber \], \(\mathrm{Fe:\:\dfrac{1.252\:mol}{1.252}}\), \(\mathrm{O:\:\dfrac{1.879\:mol}{1.252}}\), The "non-whole number" empirical formula of the compound is \(\ce{Fe_1O}_{1.5}\). Warnings Cite this Article Did you find this page helpful? These cookies will be stored in your browser only with your consent. var tr_would_you_like_to_opt_out = "Would you like to opt out from selling your personal informaion for the purpose of ads personalization? And once again, the reason grams in the numerator, grams in the denominator, The units for molar mass are, therefore, grams/mole. 1: 2: 1.008: Periodic Table.
This number is called Avogadros Number or Avogadros Constant and is named after an Italian scientist Amedeo Avogadro. Select the chemical parameter (mass, moles, molecular weight) from the list and provide it in required ones. What is the context of this Superman comic panel in which Luthor is saying "Yes, sir" to address Superman.
(a) 3 moles of carbon dioxide gas, \( CO_{2}\) Direct link to Richard's post Most chemicals will have , Posted 2 years ago. Are there any sentencing guidelines for the crimes Trump is accused of? The compound is the ionic compound iron (III) oxide. WebCalculate he number of moles you have by taking the Mass / molar mass. (a) 2 moles of Iron Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. I have seven steps to conclude a dualist reality. Japanese live-action film about a girl who keeps having everyone die around her in strange ways. How many moles of CO2 are produced when 0.300 moles of C6H12O6 are fermented? % of people told us that this article helped them. Select Mass from the drop down menu titled Calculate. a) mass of 2 moles of iron Following solved examples clearly illustrate the methods elaborated in the preceding section.
Direct link to Richard's post Sal was trying to calcula. Chemists defined the molar masses of elements such that a mole of atoms of a single element would be equal to its atomic mass, but in grams instead of u. $$ = \left(1 * 24\right) + \left(1 * 16\right) $$ Number of moles = Substance mass / Compound molar mass. $$ = 14 g $$, Calculate the mass of Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities (i.e., pure numbers), whereas molar masses have units (in this case, grams/mole). Because every glucose Due to their extremely small sizes, atoms, molecules and formula units are usually very difficult to work with. The ratios hold true on the molar level as well. WebTo calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Web1 mole of Cu = 6.022 x 10 23 atoms The mole ratios are the links between the macro and subatomic worlds. Multiply the given number of moles (2.50 mol) by the molar mass (122.548 g/mol) to get the grams. There are 8 references cited in this article, which can be found at the bottom of the page.
- [Instructor] So right over here, I have the molecular formula for glucose. So it's going to be what you the average atomic masses of carbon, hydrogen, and oxygen. six times 16 is 96.00, and this will be equal to 72, if we're just thinking Finally, divide the number of grams of the compound by the molar mass of the compound to find the number of moles. Mass of 1 Mole of \( HC_{2}H_{3}O_{2} \): We can compare the masses of all the other atoms with the mass of carbon atoms and this is the basis of the relative atomic mass scale. The relationship between the number of moles of gas, the temperature, volume and pressure. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Its main function is to facilitate childbirth, which is one of the reasons it is called the love drug or love hormone.. So, one mole of carbon with the normal isotopic mix is 12.0107 grams. Assume a \(100 \: \text{g}\) sample of the compound so that the given percentages can be directly converted into grams. On similar lines, one mole of different substances would weigh differently. The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles. WebChemists defined the molar masses of elements such that a mole of atoms of a single element would be equal to its atomic mass, but in grams instead of u. The number of moles you have of a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the compound. If your givens are 9.5, 5.07, 7.993, and 3.32, you'd round to 2 significant figures because the fewest in your givens is two in 9.5. One mole of different substances contains the same number of particles i.e.
Now, the reason why the amount Ch 15 Chemical Equillibrium Flashcards Quizlet. 18 g of Water = 1 Mole of Water \(H_{2}O\) = (\(6.02214076 x 10^23\)) number of Molecules. The cookie is used to store the user consent for the cookies in the category "Performance". Thanks. times as many carbon atoms or six moles of carbon. One mole is defined to contain exactly 6.02214076 x 1023 elementary entities (atoms, molecules, ions or electrons). And the good thing is, down Name. Well, what's that going to be?
To convert grams to moles, start by multiplying the number of atoms by the atomic weight for each element in the compound. HO) are supported and automatically converted to normal form. 72.06 divided by 180.156 is equal to, and if we round to four (Ar For Fe is 56), = number of moles relative atomic mass (Ar). By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. It does not store any personal data.
Problem: Convert 25.0 grams of KMnO4 to moles. 6.7: Mass Percent Composition from a Chemical Formula, 6.9: Calculating Molecular Formulas for Compounds, status page at https://status.libretexts.org, Identify the "given" information and what the problem is asking you to "find.". Direct link to Krish 's post Couldn't you just say And we are done. I'm not even gonna tell you its mass, but based on the molecular formula, can you figure out the In each mode, this mol calculator takes different inputs and produces a range of results as shown below: Select Moles from the drop down menu titled Calculate. We did not know exactly how many of these atoms were actually in a specific molecule.
Atoms are fundamental elements with 1 nucleus (made up of neutrons and protons), with electrons revolving around the nucleus. The degree of nesting is unlimited but all the brackets should be balanced. Molecules involve bonding between 2+ atoms, so there are 2+ nuclei in a molecule. Thanks to all authors for creating a page that has been read 830,421 times. Feel free to contact us at your convenience! ChemBuddy Chemical calculator general chemistry software. , $$ \text{Molar Mass} = \frac{Mass}{\text{Number of Moles}} $$ If we know the mass of the element then it is possible to calculate the number of moles of that element using this relationship: Related:Chemical Calculations: Relative Atomic Mass and Reacting Mass, We can calculate the number of moles of aluminium present ina) 108 g and b) 13.5 g of the element. The reason multiple names are employed for the same chemical is because each has its own advantages and disadvantages which matter differently to different groups of people. 41.304 g of NaCl 58.243 g/mol = 0.70917 moles of NaCl. Our Mole Calculator solves this equation in a split second giving accurate results and saving your time and hassle. The chemical formula of oxytocin is: C43H66N12O12S2. I have searched over internet but I couldn't find or understand it. percentage of carbon by mass of my sample? Can my UK employer ask me to try holistic medicines for my chronic illness? It also displays the molar mass of the chemical substance and details of its calculation just for reference. So we can type this into a calculator but we should remind ourselves that our final answer should have no more than four significant figures. C2HCl3O.H2O ). Since the moles of \(\ce{O}\) is still not a whole number, both moles can be multiplied by 2, while rounding to a whole number. An online mole calculator helps you to calculate the number of moles of a substance based on the molecular weight (also called molar mass) and the quantity of that material. (a) 80 g and Necessary cookies are absolutely essential for the website to function properly. Most chemicals will have both a modern scientific and older common name which are used. $$ = \frac{108}{27} = 4 Moles $$, $$ \text{Number of Moles} = \frac{Mass}{\text{Molar Mass}}$$ This number must then be multiplied by 100% to be expressed as a percent. Web A special calculator icon signals explanations of calculator use Avogadro number and mole, branches of chemistry, chemical calculations, elements and compounds particles, elements compounds and mixtures, empirical and molecular formulas, gram atomic mass molecular mass and gram formula, ions and free radicals, molecular and formula $$ \text{Molar Mass} = \frac{Mass}{\text{Number of Moles}} $$ WebThe procedure to use the molar mass calculator is as follows: Step 1: Enter the chemical compound in the respective input field Step 2: Now click the button Calculate Molar Mass to get the result Step 3: Finally, the molar mass of the chemical compound will be displayed in the new window What is Meant by Molar Mass? The cookie is used to store the user consent for the cookies in the category "Other. Direct link to 2022025's post dont u have to have to ac, Posted 3 years ago. as well as for mole (i.e. four significant figures, it would have gotten us These cookies track visitors across websites and collect information to provide customized ads. So it's going to be the So carbon's relative WebAll in One High School. Notify me of follow-up comments by email. If a compound has parentheses followed by a subscript, each element within the parentheses gets multiplied by the number in the subscript. It is found that 27 g of aluminium contains 6 1023 atoms in it. Relatively complex problems involving large amounts of masses and molar masses can be solved instantly using this moles calculator. Add this calculator to your site and lets users to perform easy calculations. Therefore, the first approach gives the correct answer, but I don't understand what you mean by 1gr/mol. Now to calculate its molar mass, we add up all of the molar masses of each atom: M = 9 12 g m o l 1 + 1 13 g m o l 1 + 14 g m o l 1 + 3 16 g m o l 1 = 183 g m o l 1 This online calculator also enables you to do the following conversions: A significant feature of this mole conversion calculator is that it also determines the number of particles (atoms, molecules etc.) You can enter a formula manually or paste the formula copied from a web page or text document (including DOC or PDF file). [ Calculators ] [ Converters ] [ Periodic Table ], Pressure Conversion Program and ideal gas law, Hardness Converter and Hardness Calculator, Design Characteristics for a Municipal Wastewater Treatment Plant Calculation, evaporation of water (lake en pan evaporation), Distributieweg 3 2645 EG Delfgauw The Netherlands Phone: +31 152 610 900 fax: +31 152 616 289 e-mail: info@lenntech.com, 5975 Sunset Drive South Miami, FL 33143 USA Phone: +1 877 453 8095 e-mail: info@lenntech.com, Level 6 - OFFICE #101-One JLT Tower Jumeirah Lake Towers Dubai - U.A.E. contained within that mass.
Definitions of molecular mass, molecular weight, molar mass and molar weight regardless of the amount.
WebThe value of the moles is equal to the number of atoms of 12 g C-12 Carbon atoms= 6.022140857 x 10^23 atoms. Web// Molar mass of Na2SO4 (Sodium Sulphate) compound float compound2 = el.u [Na]*2 + el.u [S] + el.u [O]*4; cout<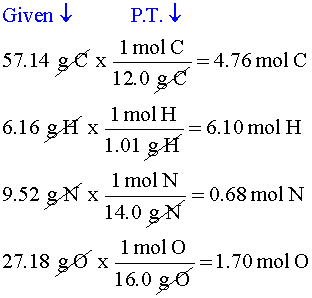 This conversion can also be done using our grams to moles calculator. For example, the relative atomic mass (Ar) of iron (Fe) is 56. A pencil and paper. Number. When did Albertus Magnus write 'On Animals'? why don't we account for constants when we check for it? Did I do that right? I still don't understand where Sal got the molar mass from and why you multiply it by the number of moles of a element; is it to convert the moles to grams? Now we have: Molar Mass of 1 Litre of water = 18.015. g, lbs, stone, oz, ton etc.) than the hundredths place, but even if we rounded over there for significant figures purposes, we would still have at least four, we'd actually have five Chemical formula (Hill notation) Molar mass (g/mol) Modify Clear % m=nM. If you have 5k $\ce{H2SO4}$ molecules, you will have 10k hydrogen atoms, not 5k. To that, we are going to add the mass of 12 moles of hydrogen. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
This conversion can also be done using our grams to moles calculator. For example, the relative atomic mass (Ar) of iron (Fe) is 56. A pencil and paper. Number. When did Albertus Magnus write 'On Animals'? why don't we account for constants when we check for it? Did I do that right? I still don't understand where Sal got the molar mass from and why you multiply it by the number of moles of a element; is it to convert the moles to grams? Now we have: Molar Mass of 1 Litre of water = 18.015. g, lbs, stone, oz, ton etc.) than the hundredths place, but even if we rounded over there for significant figures purposes, we would still have at least four, we'd actually have five Chemical formula (Hill notation) Molar mass (g/mol) Modify Clear % m=nM. If you have 5k $\ce{H2SO4}$ molecules, you will have 10k hydrogen atoms, not 5k. To that, we are going to add the mass of 12 moles of hydrogen. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Be sure to show all your steps to get full credit. If the mass of glucose is 40% then what would it be in grams? As we know, the number of protons, neutrons and electrons in atoms of different elements differ, consequently making the masses of those atoms different from each other. molecule has six carbon atoms. Could my planet be habitable (Or partially habitable) by humans? figures limiting factor. Always include the element or compound name with your answer. So this four significant figures is our significant $$ = 60 g $$, $$ \text{Number of Moles} = \frac {Mass}{\text{Molar Mass}}$$ How to convince the FAA to cancel family member's medical certificate? Parentheses ( ), square brackets [ ] and braces (curly brackets) { } can be used in the formulas. Direct link to lctx573's post Why do we have a scientif, Posted 2 years ago. we just had up here, it's going to be six moles of carbon times the molar mass of carbon, 12.01 grams per mole of carbon. Propyl acetate, C5H10O2, gives the odor and taste to pears. The cookie is used to store the user consent for the cookies in the category "Analytics". $$ = \text{number of moles} * \text{molar mass} $$ This calculator works in three different modes and these can be accessed from the drop down menu titled Calculate. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. First we have to determine the molar mass of oxytocin. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Click to share on Facebook (Opens in new window), Click to share on LinkedIn (Opens in new window), Click to share on Reddit (Opens in new window), Click to share on Twitter (Opens in new window), Click to share on Pinterest (Opens in new window), Click to share on WhatsApp (Opens in new window), Your email address will not be published. Notice that the carbon and oxygen mole numbers are the same, so you know the ratio of these two elements is 1:1 within the compound. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in the periodic table. WebTo calculate the stoichiometry of Na2(CO)3 + AgNO3 = Ag2(CO)3 + NaNO3 you must balance the equation to find the stoichiometric mole ratio of each compound. If wikiHow has helped you, please consider a small contribution to support us in helping more readers like you. percentage at the end. {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/0\/08\/Convert-Grams-to-Moles-Step-1-Version-2.jpg\/v4-460px-Convert-Grams-to-Moles-Step-1-Version-2.jpg","bigUrl":"\/images\/thumb\/0\/08\/Convert-Grams-to-Moles-Step-1-Version-2.jpg\/aid2780559-v4-728px-Convert-Grams-to-Moles-Step-1-Version-2.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a>
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/5\/5e\/Convert-Grams-to-Moles-Step-2-Version-2.jpg\/v4-460px-Convert-Grams-to-Moles-Step-2-Version-2.jpg","bigUrl":"\/images\/thumb\/5\/5e\/Convert-Grams-to-Moles-Step-2-Version-2.jpg\/aid2780559-v4-728px-Convert-Grams-to-Moles-Step-2-Version-2.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/3\/3a\/Convert-Grams-to-Moles-Step-3-Version-2.jpg\/v4-460px-Convert-Grams-to-Moles-Step-3-Version-2.jpg","bigUrl":"\/images\/thumb\/3\/3a\/Convert-Grams-to-Moles-Step-3-Version-2.jpg\/aid2780559-v4-728px-Convert-Grams-to-Moles-Step-3-Version-2.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/b\/bf\/Convert-Grams-to-Moles-Step-4-Version-2.jpg\/v4-460px-Convert-Grams-to-Moles-Step-4-Version-2.jpg","bigUrl":"\/images\/thumb\/b\/bf\/Convert-Grams-to-Moles-Step-4-Version-2.jpg\/aid2780559-v4-728px-Convert-Grams-to-Moles-Step-4-Version-2.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/1\/13\/2780559-5.jpg\/v4-460px-2780559-5.jpg","bigUrl":"\/images\/thumb\/1\/13\/2780559-5.jpg\/aid2780559-v4-728px-2780559-5.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/3\/3b\/2780559-6.jpg\/v4-460px-2780559-6.jpg","bigUrl":"\/images\/thumb\/3\/3b\/2780559-6.jpg\/aid2780559-v4-728px-2780559-6.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"moles of an element in a compound calculator
moles of an element in a compound calculator
moles of an element in a compound calculator